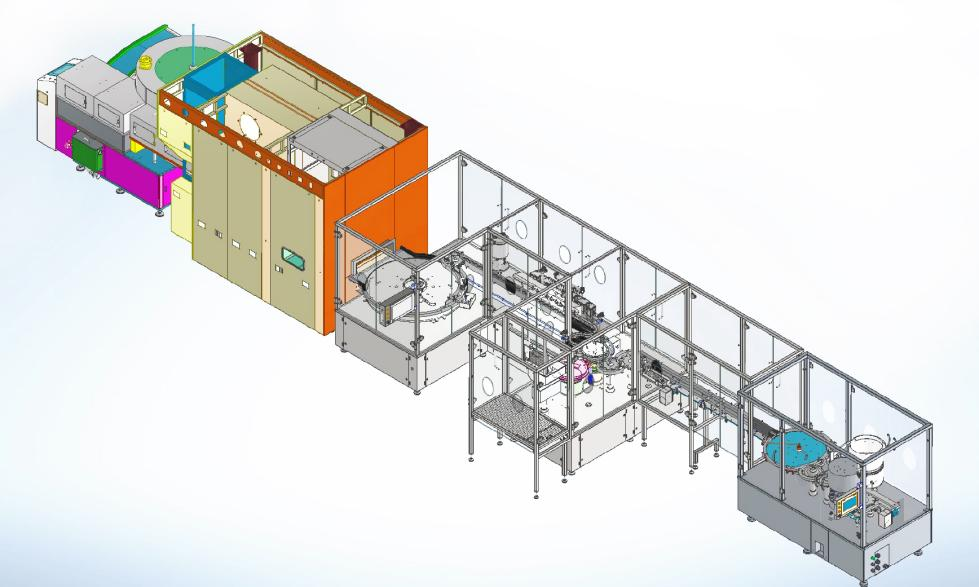
Fixed row by row system for vials:
♦ Designed for automatic vial convey from filer to freeze dryer pizza door, and convey to capper after unloading, vials pushing and pull with row by row
♦ Optional equip with sterile isolator, Rabs system
♦ Fully automatic running without manual operation
Fixed tray by tray loading system.
♦ Designed for automatic load trays into freeze dryer through pizza door from filler or dosing system (for bulk material/liquid), and convey to packing unit(for bulk material/liquid) or capper after unloaded. Trays moving row by row
♦ No change parts required, suitable for different vial size application
♦ Best solution for sterile APl or other bulk material
♦ Optional equip with sterile isolator, Rabs system
♦ Fully or semi-automatic running